On 20 November 2024, MS Nurse PRO hosted an engaging webinar, Generics and Biosimilars in the Treatment of Multiple Sclerosis (MS), featuring expert speakers Piet Eelen and Amy Perrin Ross.
The session offered valuable insights into the role of generics and biosimilars in MS care, with a focus on the nursing perspective.
If you couldn’t join us live, don’t worry! The webinar recording will soon be available on our website. Dive into the session at your convenience and explore the practical strategies and insights shared by our expert speakers. the practical strategies and insights shared by our expert speakers.
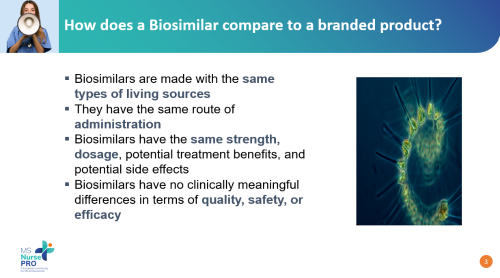
Amy Perrin Ross, APN, MSN, CNRN, MSCN, explained the key differences between biosimilars and generics, outlined their regulatory pathways, and discussed their cost implications in managing MS, one of the most expensive chronic conditions to treat. The presentation emphasised the clinical equivalence of biosimilars to branded biologics and explored the concept of interchangeability, addressing both benefits and risks.
With a focus on practical application, the session provided MS nurses with valuable knowledge to enhance patient care and optimise treatment affordability without compromising efficacy or safety.
Piet Eelen, RN, MSc, emphasised the importance of patient education, communication, and strategies for managing therapy transitions, including addressing the nocebo effect associated with biosimilars.
Piet provided examples of practical tools and approaches to support nurses in empowering patients, fostering adherence, and ensuring informed, shared decision-making. His insights reinforced the critical role of MS nurses in enhancing care quality and patient outcomes in a dynamic therapeutic environment.
Amy Perrin Ross is the Neuroscience Program Coordinator at Loyola University Medical Center in Maywood, Illinois. She is the Coordinator of the MS Center at Loyola.
Ms. Ross earned her undergraduate and graduate degrees from Loyola University in Chicago, Illinois. She has also completed coursework for her doctoral degree at the University of Illinois Medical Center. She is board certified as a Neuroscience Nurse by the American Board of Neuroscience Nursing, and as a Multiple Sclerosis Nurse by the Multiple Sclerosis Nursing International Certification Board.
Ms. Ross is an active participant in a variety of medical societies, including the American Association of Neuroscience Nurses, the American Association of Critical Care Nurses, the Multiple Sclerosis Society, the Multiple Sclerosis Association of America, and the International Organization of Multiple Sclerosis Nurses and the Consortium of MS Centers. She is a Clinical Reviewer for the International Journal of MS Care. Her work has been published in numerous journals, including Multiple Sclerosis, Frontiers in Neurology, the International Journal of MS Care, the International Journal of Clinical Practice, Neurology, Practical Neurology and the Journal of Neuroscience Nursing. Ms. Ross has participated in more than 40 research studies and has been invited to speak before numerous national and international meetings and conferences. She has been honored for her work in the field, receiving the 2015 June Halper Award of Excellence in International MS Nursing, recognizing her for leadership and creativity in the care of people with MS and their families.
Piet Eelen is a Clinical Nurse Specialist Multiple Sclerosis working in the National Multiple Sclerosis Center (NMSC) of Melsbroek, Belgium. He has a post-bachelor degree in Rehabilitation and Continence Nursing. From 1990 until 2011 he worked as a head nurse of a rehabilitation ward. Since 2012 he works as a clinical nurse specialist guiding and supporting persons with MS in his clinical practice, with a special focus on chronic disease management and lifestyle factors in MS.
He organizes courses for nurses to stimulate development and education, in the NMSC and in Belgium. He participates and collaborates with national and international healthcare associations. He is an invited speaker and member of advisory boards organized by pharma companies. Finally he participates and organizes research projects in MS in the NMSC. Since 2012 he is the president of the Belgian Association of MS-Nurses. Since 2020 he is the chair of the syllabus committee of MS Nurse PRO.
This webinar was supported by Sandoz, with no involvement in the development of the scientific program or presentations.

All Blogposts, e-learning Courses, e-Newsletters, e-Newsflashes and the website content of MS Nurse PRO is originally created in English (UK).
Our Educational Read Blogposts and our e-learning courses are reviewed by our scientific committee on accuracy and objectiveness.
Next, the content is auto-translated by Microsoft Translator and made available on our platform.
The translated content is not language reviewed with the exception of our e-learning Course content.
MS Nurse PRO has put a process in place to have our e-learning Courses language reviewed by native speaking experts (nurses or neurologists).
We make all translated e-learning Courses available immediately and next, the language review process is started. This review process can take several months.