High-dose vitamin D (cholecalciferol) monotherapy significantly reduced disease activity in patients with clinically isolated syndrome (CIS) and early relapsing-remitting multiple sclerosis (RRMS) according to results of the D-Lay MS clinical trial (NCT01817166) published in JAMA in March 2025.2 Conducted across 36 centres in France from 2013 to 2020, this study provides evidence supporting vitamin D as a therapeutic option for CIS and early-stage RRMS.
The Phase 3 trial was designed to see if high-dose cholecalciferol (or vitamin D3) could prevent MS conversion from CIS.
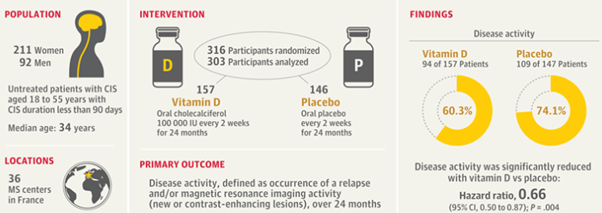
A total of 303 adults aged between 18 and 55 years, who’d had a CIS episode in the last three months, weren’t receiving any disease-modifying therapy (DMT), and had serum vitamin D levels less than 100 nmol/L were randomly assigned to receive oral high-dose cholecalciferol (100,000 International Units [IU]) or placebo once every two weeks until conversion to MS occurred, or for a maximum of two years.
Disease activity, defined as a new or enlarging lesion, an active inflammatory lesion identified on MRI scans, or a relapse was significantly reduced in patients on vitamin D compared with placebo (60.3% vs 74.1% respectively, p=0.004). This equates to a 34% lower risk of disease activity for patients receiving vitamin D compared with those on placebo.
The average time to the onset of new disease activity was nearly twice as long in the vitamin D group as in the placebo group (432 vs. 224 days), which was a statistically significant difference.
All possible signs of MRI disease activity occurred less frequently in the vitamin D group than in the placebo group, including overall MRI activity (57.1% vs. 65.3%), new or enlarging lesions (46.2% vs. 59.2%), and active inflammatory lesions (18.6% vs. 34%).
No significant differences were observed with relapses, which occurred in 17.9% of the vitamin D group and 21.8% of the placebo group. Vitamin D also didn’t have a significant impact on measures of disability, fatigue, depression, anxiety, or quality of life.
The findings from the D-Lay study contrast with those of the PrevANZ trial, published in 2024, which also focused on people with CIS.3 Participants took daily doses of vitamin D3 (1,000, 5,000, or 10,000 IU) or a placebo for 48 weeks. The results showed that vitamin D did not significantly reduce the progression from CIS to MS compared to the placebo group. Around 58% of participants developed MS within the study period, and the rates were similar across all groups. The low dose vitamin D (1000 IU/day) was as effective as the higher doses.
There are some key differences between the studies which should be considered when trying to understand the different outcomes:4
While vitamin D deficiency is associated with increased MS risk and relapses, it is not a standalone treatment for MS. The studies described above highlight that vitamin D alone has, at best, a small benefit, but does not replace the need for other therapies that are specifically designed to reduce relapses, new lesions, and slow MS progression.
People with MS should be advised to keep in regular communication with their MS care team, who can provide personalised guidance and help to integrate vitamin D into a broader plan that includes medications, lifestyle adjustments, and other treatments designed to improve quality of life and manage symptoms effectively.